Association of Food and Drug Officials
125th Anniversary
AFDO celebrated its 125th anniversary in 2021. In honor of the occasion, a video history was produced. Watch the 27-minute video, called “Milestones in AFDO History,” here.
Association of Food and Drug Officials
Conversations In Food Safety Series
In September of 2024, AFDO premiered a series of interviews with key food safety influencers conducted by and featuring AFDO Senior Advisor Joe Corby. These interviews are featured below:
- First interview can be found here (Reed)
- Second interview can be found here (Fruin)
- Third interview can be found here (Miles)
- Fourth interview can be found here (Corby)
From early colonial times, most of the food and drug regulations in this country were enacted at the state and local level.
These regulations were generally targeted toward specific food products and generally for quality concerns. In 1641 Massachusetts introduced its first food adulteration law, which required the official inspection of beef, pork and fish. This was followed in the 1650’s with a regulation for the quality of bread. Virginia also enacted laws to regulate weights and measures for corn and to outlaw the sale of adulterated wines.
During the latter half of the nineteenth century, the scale and scope of state level food regulation expanded considerably, in part for the following reasons:
- Households became more dependent on food purchased in commercial markets. While this increased the variety of foods available, it also increased uncertainty about food quality.
- Technological change in food manufacturing gave rise to new products and increased product complexity. New food products included alum-based baking powders, oleomargarine, glucose, canned foods, blended whiskey, and foods containing chemical preservatives, Unfamiliarity with these new products generated consumer concerns about food safety and food adulteration.
- The rise of analytic chemistry facilitated the “cheapening” of food in ways that were difficult for consumers to detect. For instance, the introduction of preservatives made it possible for food manufacturers to mask food deterioration. Additionally, the development of glucose as a cheap alternative to sugar facilitated deception on the part of producers of high priced products like maple syrup. Hence, concerns about adulteration were increasingly felt and there was a growing perception that regulation was necessary.
The following chronology describes some of the notable events and milestones that impacted the history of the Association of Food and Drug Officials (AFDO).
1862
President Abraham Lincoln appoints a chemist, Charles M. Wetherill, to serve in the new Department of Agriculture. This was the beginning of the Bureau of Chemistry, the predecessor of the Food and Drug Administration.

1880
Peter Collier, chief chemist, U.S. Department of Agriculture, recommends passage of a national food and drug law, following his own food adulteration investigations. The bill is defeated, but during the next 25 years more than 100 food and drug bills are introduced in Congress.
1883
Dr. Harvey W. Wiley becomes chief chemist, expanding the Bureau of Chemistry’s food adulteration studies. Campaigning for a federal law, Dr. Wiley is called the “Crusading Chemist” and “Father of the Pure Food and Drugs Act.”
1896
Joseph Blackburn, the Food and Dairy Commissioner for Ohio, meets with his counterpart from Michigan, Elliot Grosvenor in Toledo, Ohio, to develop the foundation for an organization whose mission will be defined by the promotion of regulatory uniformity. The initial meeting of the National Association of State Dairy and Food Departments, which later became the Association of Food and Drug Officials, will occur the following year on August 25, 1897, in the Turkish Room of the Cadillac Hotel in Detroit, MI. Representatives attended this meeting from the following ten States: Colorado, Connecticut, Iowa, Massachusetts, Michigan, Minnesota, New York, Ohio, Pennsylvania, and Wisconsin.



Uniformity in legislation and rulings relative to dairy and food products is a major objective of the association from the very beginning. Two other well recognized objectives are the promotion of legislation that would protect public health and the harmonization of interests represented by those charged with the enforcement of state food and drug laws.
Thus began a nationwide professional association combining the best talents of government officials at all levels, industry, academia, and consumers
1897
Important
The association’s name changed to the National Association of State Dairy and Food Departments.
1902
The first few years of the Association would focus on developing a constitution and by-laws, which are finally completed and approved at the 1902 convention in Portland, Oregon.
At this convention. Commissioner Alfred H. Jones from Illinois suggested the need for the formation of a national food commission and a Commissioner to lead it. The idea for this commission would eventually come to pass and lead to the establishment the Food & Drug Administration.

This convention also marked the beginning of the association’s discussions of a proposed Pure Food & Drug Act being promoted by Dr. Harvey Wiley, Chief Chemist with the Bureau of Chemistry of the United States Department of Agriculture. The association strongly supported national legislation and approved a motion to send their entire Legislative Committee to Washington, D.C. to appear before the Senate Committee debating the proposed legislation. The Association would, also, pass Resolutions in 1904 and 1905 supporting the national bill and sends an extensive telegram to President Theodore Roosevelt in 1905 indicating its full support as well.

Dr. Wiley would obtain $5,000 in funding from the U.S. Congress and in 1902 conducts research with human volunteer subjects that would become known as the “Poison Squad”. Dr. Wiley, had spent years researching mislabeled food, and realized that consumers had no idea what they were consuming and no one knew the long term effects of these additives. So he gathered the “Poison Squad,” a group of healthy young men who voluntarily consumed poisoned food so that Dr. Wiley could examine the effects of chemicals and adulterated food on them. They became a pop culture sensation, inspiring poems and minstrel shows. And eventually, their work helped to bring about the Pure Food and Drug Act, which would eventually lead to the creation of the FDA. Women banded together, notably in the Federated Women’s Clubs, for political clout. Major canners became supporters of the legislation and voluntarily abandoned the use of questionable chemicals.
Dr. Wiley would praise the Women’s Clubs and our state association for pressuring President Roosevelt and Congress to pass a national food law. One of the heroes of the pure food and drug movement was Commissioner Edwin F. Ladd from North Dakota who would become the association’s President in 1908.

1905
Important
Association name changed to Association of State and National Food and Dairy Departments
1906
The food and drug bill finally passed and the country had its first comprehensive Pure Food and Drug Act. Dr. Wiley spoke at the association’s meeting in Hartford, Connecticut that same year just 2 ½ weeks after the law was enacted and thanked those who worked to accomplish the national rule. Here is what Dr. Wiley would say:
“The people of this country, it seems to me, are to be congratulated upon the splendid work which has been accomplished by the national legislature. While no one claims that the law which has been enacted is a perfect one, yet everyone will admit that it is based on sound principles of ethics and justice. If experience shall show that it is weak in any of its provisions, the attitude of the national Congress is such to this great question that any needed amendments can be easily secured. This meeting, therefore, takes place under what may be considered most favorable auspices respecting the legislative aspects of the pure food question.”
1909
The association’s convention in 1909 is known as the “Battle in Denver”. Two very divisive issues were debated which split state delegates evenly. The first was consideration for developing a model state food law which supporters believed would promote uniformity among the states. Opposing states indicated the new federal rule passed in 1906 was sufficient and states should model that law for any state rule. It took 18 years to resolve this issue.
The second issue that caused huge uproar was the safety of benzoate of soda as a preservative in foods. Dr. Wiley was strongly opposed to the use of benzoate of soda in food as were half of the state delegates present at the convention. The other half of state delegates supported the opinions of industry and other scientists who traveled to the conference to join in the debate. President Theodore Roosevelt had asked Secretary of Agriculture James Wilson to appoint a Referee Board of scientists to evaluate the issue. The Referee Board exonerated benzoate of soda which infuriated Dr. Wiley.

The argument carried over into the election of the association’s next President. George L. Flanders from New York was the candidate of USDA Secretary Wilson while A. C. Bird of Michigan was the candidate of Dr. Wiley. The state votes were split right down the middle with 18 for Flanders and 18 for Bird. The deciding vote would be delivered by USDA which in previous years had been provided for Dr. Wiley. At the last minute however, Secretary Wilson stepped in and informed Dr. Wiley that he and not Dr. Wiley would vote on behalf of USDA. Flanders would, therefore, win and Dr. Wiley was humiliated again.

1911
The Northeast Food & Drug Officials Association (NEFDOA) is established as a regional affiliate of the Association of Food and Drug Officials (AFDO) and represents the states of Connecticut, Maine, Massachusetts, New Hampshire, New York, Rhode Island and Vermont, the Atlantic Provinces, New Brunswick, Newfoundland, Nova Scotia, and Prince Edward Island, Ontario, and Quebec in Canada.
1912
Important
Association name changed to Association of American Dairy, Food, and Drug Officials
1913
The association passes a motion recommending the formation of an Office of State Cooperation which the federal government approves. This was the beginning of what would eventually become FDA’s Division of Federal State Relations and today the FDA Office of Partnerships. These offices have solidified relationships between FDA and the states for many years.
1916
The Central Atlantic States Association of Food & Drug Officials (CASA) is organized as a regional affiliate of the Association of Food and Drug Officials (AFDO) and represents the states of Virginia, West Virginia, Maryland, Delaware, Ohio, Pennsylvania, New Jersey, and New York, the District of Columbia, and the Province of Ontario, Canada. CASA would eventually grow to be comprised of eight local conferences: Virginia, Susquehanna, Pittsburg, Niagara Frontier, North East New York, New York, Philadelphia, and Baltimore.
1924
Important
Association name changed to Association of Dairy, Food, and Drug Officials of the United States
1927
After years of debate, the Association would finally produce a ‘Model Uniform Food Law’ which many states would go on to adopt. This model act was designed to prevent the manufacture, possession, sale or delivery of adulterated or misbranded food and drugs. It set specific standards for determining when food and drugs are considered adulterated or misbranded.
1937
In 1937, a Tennessee drug manufacturer began to market a liquid sulfa drug called Elixir Sulfanilamide. Unfortunately, the solvent used in producing this drug was a highly toxic variant of antifreeze and over 100 people died from taking this drug. Many of these deaths were children and a public outcry occurred in this country. The Association passed a Resolution reaffirming its position in support of passage of a stronger Food, Drug, and Cosmetic Act. Here is what the Resolution said:
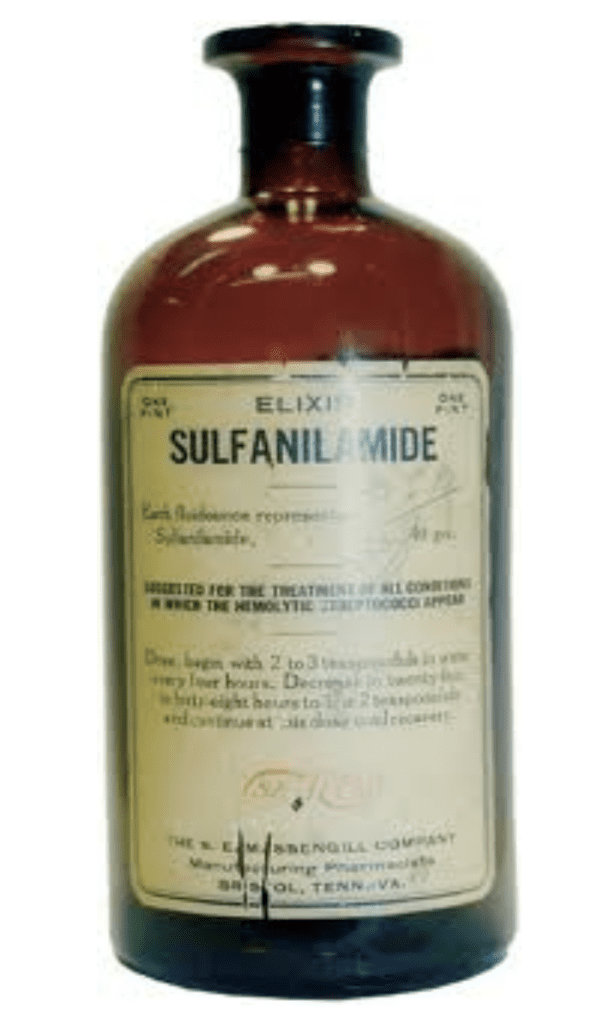
“Whereas, the recent much publicized disaster in the poisoning and death of over 100 persons from the unsupervised distribution of an unknown and untested proprietary remedy might have been prevented had there been enacted in the past an adequate and strong federal food and drugs act, providing for licensing, and control of dangerous drugs, Therefore Be it Resolved , that the association reaffirm its position taken at previous meetings in the support of the passage by Congress of a strong and adequate food, drug, and cosmetic law”
1938
In 1938 President Franklin Roosevelt would sign into law a new and stronger Food Drug & Cosmetic Act. Under the 1938 law, the FDA was given considerably greater authority over the food and drug industry.

Important
Association name changed to Association of Food and Drug Officials of the United States
1939
In 1939 the Association passed a Resolution to reaffirm its strong support of uniformity and support for state adoption of uniform food, drug, and cosmetic legislation. While the Association would continue to support uniformity, it always believed that states should have the authority to act in the absence of federal action. It further acknowledged that the overwhelming majority of food safety work performed in this country has been and will always be performed at the state and local government level.
AFDO’s Vice President E. G. Woodward from Connecticut wrote an editorial in AFDO’s Quarterly Bulletin where he spoke of an integrated food safety system as follows:
“The greatest single program of work before the Association is to follow through with its efforts for uniformity in state legislation until this whole nation has an integrated system of similar Food, Drug and Cosmetic Laws, interpreted, administered, and enforced in a single spirit of uniformity.”
1949
After years of discussion, an AFDO Inspectors Manual Committee was organized to develop an Inspectors Manual to standardize field inspection and investigation activities. The manual originally contained 15 sections that addressed both food and drug inspection, sampling, investigation, and field testing procedures. It would become the model for current day Inspector Manuals used by federal and state officials.
1962
The association recommended that FDA provide for grants to states needing financial assistance or personnel to properly operate a food or drug regulatory program. FDA would eventually provide states with inspection grants. AFDO’s President Carroll Brinsfield stated the following:
“The U.S. Public Health Service aids states in environmental sanitation work, so why shouldn’t Food and Drugs provide assistance in their field?”
1965
One of the highlights of the 1965 conference was the release of the completed Inspector’s Manual. FDA participated with AFDO officials to complete the effort.
1968
Reorganization of federal health programs would place FDA into the Public Health Service. This would form the basis for the development of Cooperative Programs In the following year,1969, FDA began administering sanitation programs for milk, shellfish, food service, and interstate travel facilities, and for preventing poisoning and accidents. These responsibilities were transferred from other units of the Public Health Service which FDA had become part of in the previous year.
1973
Newly inaugurated FDA contracts with state agency programs become one of the most important innovations in FDA-State programs to be developed. The implementation of contracts for food inspection, medicated feed mills, interstate travel and food sanitation, and food surveillance sample analysis resulted in greater joint consumer protection, improved federal-state cooperation, and enhanced the versatility and capability of all participating agencies.
The Consumer Product Safety Commission is created by Congress. The Commission would take over programs pioneered by FDA under the 1927 Caustic Poison Act, 1960 Federal Hazardous Substances Labeling Act, 1966 Child Protection Act, and Public Health Services accident prevention activities for the safety of toys, home appliances, and other consumer products. State consumer product safety officials became affiliated with AFDO.
1974
AFDO President Frank Fischer from Indiana discussed the matter of federal preemption which became a primary issue with the association for many years. President Fischer stated the following:
“A matter of ongoing concern to many state officials is the increasing discussion concerning federal preemption in the area of food and drug laws. At this time there are several bills in the Congress which would pre-empt all labeling requirements of food products.”
Throughout the year, the issue of state preemption became such an important discussion point, that the association formed an Ad-Hoc Committee on Federal Preemption of State Food and Drug Acts. The association have fought very strongly against a number of preemption bills that were proposed in Congress for a number of years.
FDA began publishing and distributing the Directory of State Officials. It would be more than 30 years later that AFDO would take over the operation of the Directory of State & Local Officials (DSLO) under a Cooperative Agreement with FDA.
AFDOUS, as we were then known, became a world-wide association by changing the by-laws to allow food and drug officials from any nation to qualify as regular members. The same is true for food and drug industry representatives of all nations being eligible to qualify as associate members. As a result of the by-law change, the new name of our association became: “Association of Food and Drug Officials” (AFDO). This amendment would also allow FDA officials to become members of AFDO.
Important
Association name changed to Association of Food and Drug Officials.
1980
AFDO publishes a position paper on uniformity of laws and regulations that concluded with the following statement:
AFDO contends that federal preemption should not be imposed when states have requirements “in addition to” federal requirements. AFDO unanimously agrees that state requirements and programs will closely parallel federal ones but the states cannot be prevented from the occasional provisions of requirements “in addition to” federal requirements when the citizens of a state desire additional health protection. In an effort to achieve necessary consumer protection, AFDO will oppose federal legislation containing preemptive provisions.
1981
AFDO has erected a historical landmark for Dr. Harvey Wiley in his hometown of Kent, Indiana. The historical marker is placed at the SW corner of State Highway 256 & County Route 850 West, in Kent, Indiana; Jefferson County.

1985
AFDO obtains a national listing by the Council of State Governments of its 1984 Model Uniform Food, Drug, and Cosmetic Act. The model act was presented and available to each State Legislature by the Council.
1988
The Food and Drug Administration Act of 1988 officially established FDA as an agency of the Department of Health and Human Services with a Commissioner of Food and Drugs appointed by the President with the advice and consent of the Senate. The Act broadly spelled out the responsibilities of the Secretary and the Commissioner for research, enforcement, education, and information.
1990
The Nutrition Labeling and Education Act was passed and required all packaged foods to bear nutrition labeling and all health claims for foods to be consistent with terms defined by the Secretary of Health and Human Services. The law preempted state requirements about food standards, nutrition labeling, and health claims and, for the first time, authorized some health claims for foods. The food ingredient panel, serving sizes, and terms such as “low fat” and “light” became standardized. AFDO organized a host of national meetings to train state officials on the new labeling law.
1992
In 1992, following a meeting in Atlanta, GA, between FDA officials and representatives of AFDO, a “white paper” was prepared that advocated expansion of leveraging opportunities between the states and FDA. This developed into approximately 165 partnerships and work-share agreements between the states and FDA.
1993
The AFDO Endowment Foundation is established by the Board of Directors with the express purpose of raising a permanent endowment fund, and to provide support to AFDO’s educational and scientific mission. Separated from the general operating budget, the endowment fund is administered by its own Board of Trustees consisting of 10 AFDO members from the private sector or former government employees.
1994
The Seafood HACCP Alliance was initiated by the Association of Food and Drug Officials (AFDO) and their regional affiliate of Southern States (AFDOSS) in conjunction with a cadre of Sea Grant Seafood Specialists which originally assisted the National Fisheries Institute (NFI) with their initial HACCP training programs. The first formal Alliance meeting, June 22-23, 1994 in Portland, Maine established a project ‘Steering Committee’ largely based on the contributing authors for the original proposal. This Committee includes members representing the three principal federal agencies; Food & Drug Administration, US Department of Agriculture, and National Marine Fisheries Service, the various state agency organizations through AFDO regional affiliates and the Interstate Shellfish Sanitation Conference, and the industry trade associations, NFI and Food Products Association (formerly National Food Processors Association). The proposed approach recognized the essential role of state regulatory authorities, the educational network of Sea Grant and Cooperative Extension Service, and the need for regional attention per seafood diversity.

1996
In 1996, well-known food law attorney and avid AFDO member George M. Burditt compiled a commemorative history of AFDO in his book entitled “AFDO The First Hundred Years.” It chronicles most all of what is written here up to 1996. In the conclusion of this book, Mr. Burditt states the following:
“The benefits of the Association to society are incalculable. Uniformity – a key word from the very beginning in 1897, and now the first word in AFDO’s slogan – is the crowning achievement. Exchange of scientific information, sharing of compliance policies, procedures and problems, and helping one another to keep current in a constantly advancing field of knowledge are among the themes so apparent to a student of the history of the Association.
The second operative word in the Association’s slogan is Cooperation. For the first few years of the Association’s history, it was cooperation among state officials only. Then cooperation with the federal government was added. And finally, cooperation with industry became an integral part of the Association’s program. Cooperation is not only in the Association’s slogan but is at the very heart of the organization.”
AFDO celebrates its 100th year at its Annual Conference in San Francisco.
1997
AFDO President Dan Smyly, Florida Department of Agriculture & Consumer Services, addresses the Regulatory Affairs Professional Society Annual Conference held in Washington, DC, and the U.S. Department of Agriculture (USDA) Food Safety and Inspection Service Annual Federal/State Conference on Food Safety in Sacramento, CA, where he advises attendees of AFDO’s vision of a national integrated food safety system (IFSS). Smyly requested support from USDA and FDA to convene a meeting of a select group to develop the blueprint for a vertically integrated national food regulatory system.
AFDO officials had been discussing this idea for a while and the design for the proposed system had actually been drawn out on a cocktail napkin that has been preserved and is currently displayed in AFDO’s office.
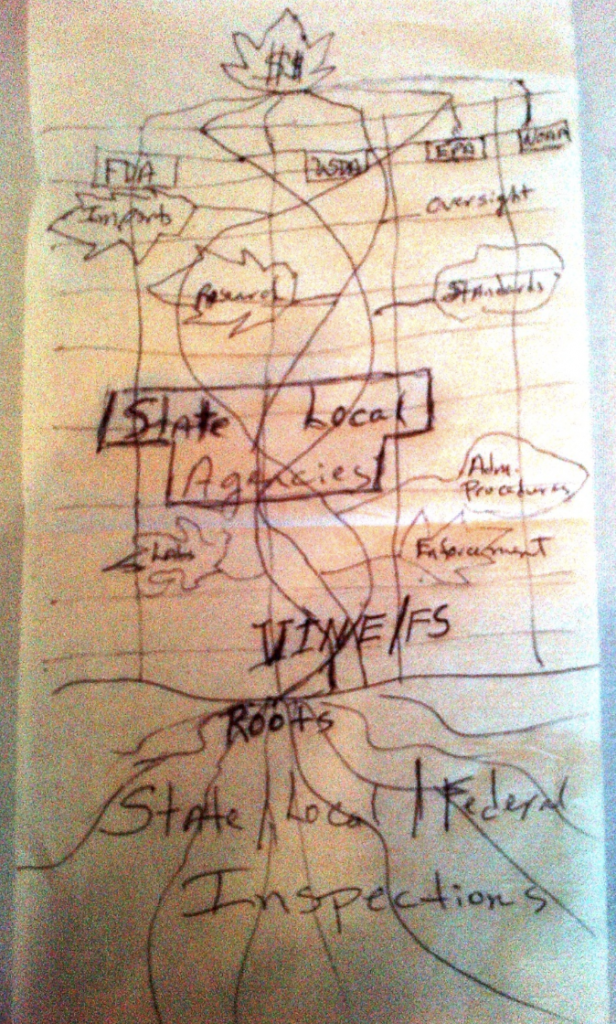
1998
The National Food Safety System (NFSS) is organized to advance the vision of an integrated food safety system. Through this project, FDA and USDA Food Safety and Inspection Service (FSIS) actively began engaging state and local food safety counterparts. CDC and EPA also began to explore new approaches for partnering on food protection. In September 1998, a meeting was held for food safety officials from FDA, USDA-FSIS and CDC along with the food safety agencies from all 50 states. This historic meeting produced a broad consensus on the need to meet challenges together to integrate food safety activities at all levels of government. Attendees discussed their visions of a successful food safety system, identified obstacles to achieving that vision and proposed action items to remove those barriers.
NFSS work groups were formed, consisting of individuals from the federal agencies as well as state and local officials. Under the guidance of a multi-agency steering committee, these work groups were charged with generating ideas to promote an IFSS. There were five initial NFSS work groups: Roles and Responsibilities; Outbreak Coordination and Investigation; Laboratory Operations and Coordination; Information Sharing and Data Collection; National Uniform Criteria.
2000
The Department of Health and Human Services; Office of Inspector General evaluates the Food and Drug Administration’s oversight of food firm inspections conducted by States through contracts and partnership agreements. A report is issued entitled FDA Oversight of State Food Firm Inspections a Call for Greater Accountability
2001
The first AFDO State Resource Survey is conducted and released. One of the primary objectives of this survey was to illustrate the extent of food safety regulatory activities conducted by state and local government agencies. The survey provides a limited and conservative picture of these food protection activities. Some of the results recorded in the 2001 survey included 2,500,757 food safety inspections performed, 328,068 food samples collected and tested, and 128,430 enforcement actions taken against violative firms. The survey would be conducted again in 2003 and 2008.
2006
A “Basics of Applications of Inspection and Investigation Course” is developed as a result of funding provided by a “States Helping States” cooperative agreement between AFDO from the Centers for Disease Control and Prevention (CDC). This grant would also set up mentoring opportunities for states to receive assistance from other states on a host of food protection practices.
2007
AFDO assumes responsibility for the updating and management of FDA’s Directory of State & Local Officials (DSLO). The directory becomes the primary contact resource for regulatory officials and a key communication development for integrating the food safety system. The directory is updated twice a year to assure accuracy.
2008
AFDO participates in a George Washington University and Robert Wood Johnson Foundation project to describe in detail the history of previous efforts to develop a national, integrated food safety system, and provide specific recommendations for achieving that concept with emphasis on the role of state and local agencies.
The U.S. Food and Drug Administration (FDA) establishes a Rapid Response Teams (RRTs) Program initiative that partners with state programs to build food safety infrastructure and a concept of integrated rapid response for all-hazards human and animal food emergencies.
AFDO receives a Kellogg Foundation grant to work with Michigan State officials to establish a training academy for food protection officials. AFDO would soon establish the infrastructure of an entity that would become the International Food Protection Training Institute (IFPTI). In addition to establishing this institute, AFDO identified the need for and assisted in the development of a “Fellowship in Food Protection” program to train future food safety leaders in the concept of an integrated food safety system.

2009
The Partnership for Food Protection (PFP) is established to help implement the recommendations from the 2008 50-State Workshop. The PFP will be designated the primary group to advance and build the IFSS.
FDA sets a strategic vision in a draft entitled “Establishing a Fully Integrated National Food Safety System with Strengthened Inspection, Laboratory and Response Capacity”.

The Association of Food and Drug Officials (AFDO) developed a Model Code for Produce Safety in response to a growing number of outbreaks associated with consumption of fresh fruits and vegetables
AFDO participates in efforts to establish regulatory program standards for manufactured food and retail food. The adoption and implementation of these standards are promoted as a critical step towards implementing an integrated national food safety system. In addition to these existing standards, consideration will be given to the need for additional program standards in order to adequately cover the entire food supply chain.
2010
Produce Safety Alliance is established through a cooperative agreement between Cornell University, USDA, and FDA is completed.

IFPTI begins the development of a 3-week Fellows for Food Protection program. Participants are solicited from state and local agencies throughout the U.S. and selected fellows begin the first original program in August. The Fellows selected year-long research projects and will present the results of their work during a poster session at the 2011 AFDO Conference in Plano, Texas
2011
In 2011, President Barack Obama signed into law the Food Safety Modernization Act (FSMA) that supports an integrated food safety system. In general terms, the law directs a more pro-active approach to food safety, rather than primarily reacting to food safety problems, and a more “science-based” approach to food safety concerns. AFDO continues to work with all stakeholders to advance what was its original vision for a fully national integrated food safety system.

The Food Safety Preventive Controls Alliance (FSPCA) is established in late 2011 by a grant from the U.S. Food and Drug Administration (FDA) to Illinois Institute of Technology’s Institute for Food Safety and Health (IIT IFSH).

The Association of Food and Drug Officials (AFDO) proposes the creation of an Alliance of State Manufactured Food Program Managers to advance a national integrated food safety system. The Manufactured Food Regulatory Program Alliance (MFRPA) is formed under the “Alliance for Advancing a National Integrated Food Safety System Cooperative Agreements Program”.

2014
The Retail Program Standards Grant Program is established as a partnership between the U.S. Food and Drug Administration (FDA) and the Association of Food and Drug Officials (AFDO). The program provides funds for the completion of projects and training to enhance conformance with the Voluntary National Retail Food Regulatory Program Standards (Retail Program Standards).
2015
FDA finalizes several regulations to implement the FDA Food Safety Modernization Act (FSMA), as listed below:
- Preventive Controls for Human Food Final Rule
- Preventive Controls for Food for Animals Final Rule
- Standards for Produce Safety Final Rule
- Foreign Supplier Verification Programs (FSVP) for Importers of Food for Humans and Animals Final Rule
- Accredited Third-Party Certification Final Rule
- Sanitary Transportation of Human and Animal Food Final Rule
- Mitigation Strategies to Protect Food Against Intentional Adulteration Final Rule

2017
The U.S. Food and Drug Administration (FDA) in collaboration with the National Conference on Interstate Milk Shipments (NCIMS), the Interstate Shellfish Sanitation Conference (ISSC), and the Association of Food and Drug Officials (AFDO), partnered to provide State Cooperative Program Grants for the Grade “A” Milk Safety and National Shellfish Sanitation Programs. Grant funding through AFDO became available to state or territorial (Puerto Rico) agencies that have the regulatory authority to implement the Grade “A” Milk Safety Program and/or the National Shellfish Sanitation Program.
2018
A collaboration known as “Partners with A Common Purpose” (PwCP) is formally launched in 2018. Industry food safety and quality assurance professionals and food protection regulatory professionals engage and participate in face-to-face discussions within a safe-harbor forum to investigate, explore, discuss and advance particular topics as they relate to food safety within both the industry and regulatory arenas.

2020
AFDO sponsors a stakeholders meeting to discuss strategies for reducing foodborne illness to meet the goals of Healthy People 2030. Strategies identified were shared with the U.S. Congress. To reduce an estimated 48 million cases of foodborne illnesses, 128,000 hospitalizations and an estimated $15.6 billion per year in health care and other costs, stakeholders recommended the following funding was needed:
- CDC Food Safety – $20 million (Half to States)
- FDA Food Safety – $20 million (Half to States)
2024
Conversations in Food Safety Series: Food safety education is important 365 days a year, but September is the month when many organizations, AFDO included, put a spotlight on what happens all year. This year also marked the 26th birthday of the Integrated Food Safety System.
To celebrate these milestones, AFDO shared a series of interviews with key influencers conducted by and featuring AFDO Senior Advisor Joe Corby.
- The first Conversations in Food Safety guest was Mark Reed, a food safety program manager with the Kentucky Department for Public Health. Reed has held many leadership roles in food safety for both government and academia. He began his career as a field technical consultant for the Kentucky Department for Public Health. From 2016 to 2018, he was the project manager for the then newly created national curriculum standards grant with AFDO. Reed also taught courses as an adjunct professor at Eastern Kentucky University from 2007 to 2011.
- The second Conversations in Food Safety guest was John Fruin. Fruin has a background in agriculture and veterinary sciences. He began his extensive 30-year career with the U.S. Army as a veterinary corps officer. He also worked with the Florida Department of Agriculture, supervising inspectors in both food and meat programs while meat was inspected at the state level. Fruin has collaborated with Joe Corby to put together a guidance document for custom processing.
- The third Conversations in Food Safety guest was AFDO past president Pamela Miles. Miles’ background is in agriculture and food science. She graduated from Purdue University with a bachelor’s degree in food science. Miles has experience in both the private and public sectors, first working at a dairy plant in Indiana and an ice cream plant in Virginia. She then worked with the Virginia Department of Agriculture and Consumer Science (VDACS) as an inspector, then transitioned into a regional supervisor position, and was promoted to program manager. Miles is now the program manager for the Office of Dairy & Foods program within VDACS.
- The final Conversations in Food Safety guest was Joe Corby. Corby is both a former president and former executive director of AFDO. With a background in environmental health, he started his career as a food inspector with the New York State Department of Agriculture and Markets and gradually worked his way up to a regional supervisor position within the agency. Corby also worked with Dan Smyly, another past president of AFDO, helping to create the road map toward the Integrated Food Safety System. He remains a mentor to many food safety leaders and continues to be a strong advocate for food safety today.


